PDT(Photodynamic Theraphy)
Treat What Happended Yesterday ?
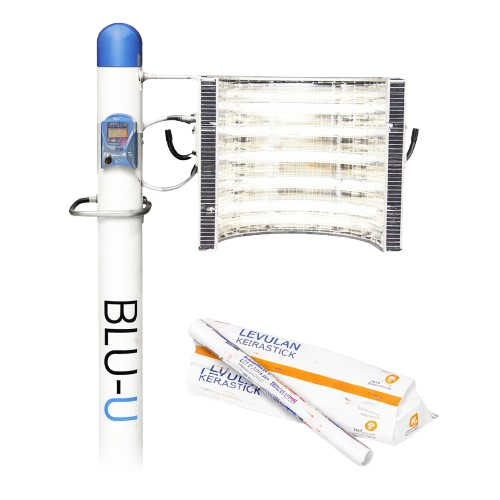
LEVULANR KERASTICK® (aminolevulinic acid HCI) is a prescription medicine used on the skin with blue light treatment (BLU-U® Blue Light Photodynamic Therapy or PDT) for the treatment of minimally to moderately thick actinic keratoses (AK's) of the face, scalp or upper arms. It is for use only as an in-office treatment. LEVULAN KERASTICK treatment is given by a healthcare provider only and is not for use at home.
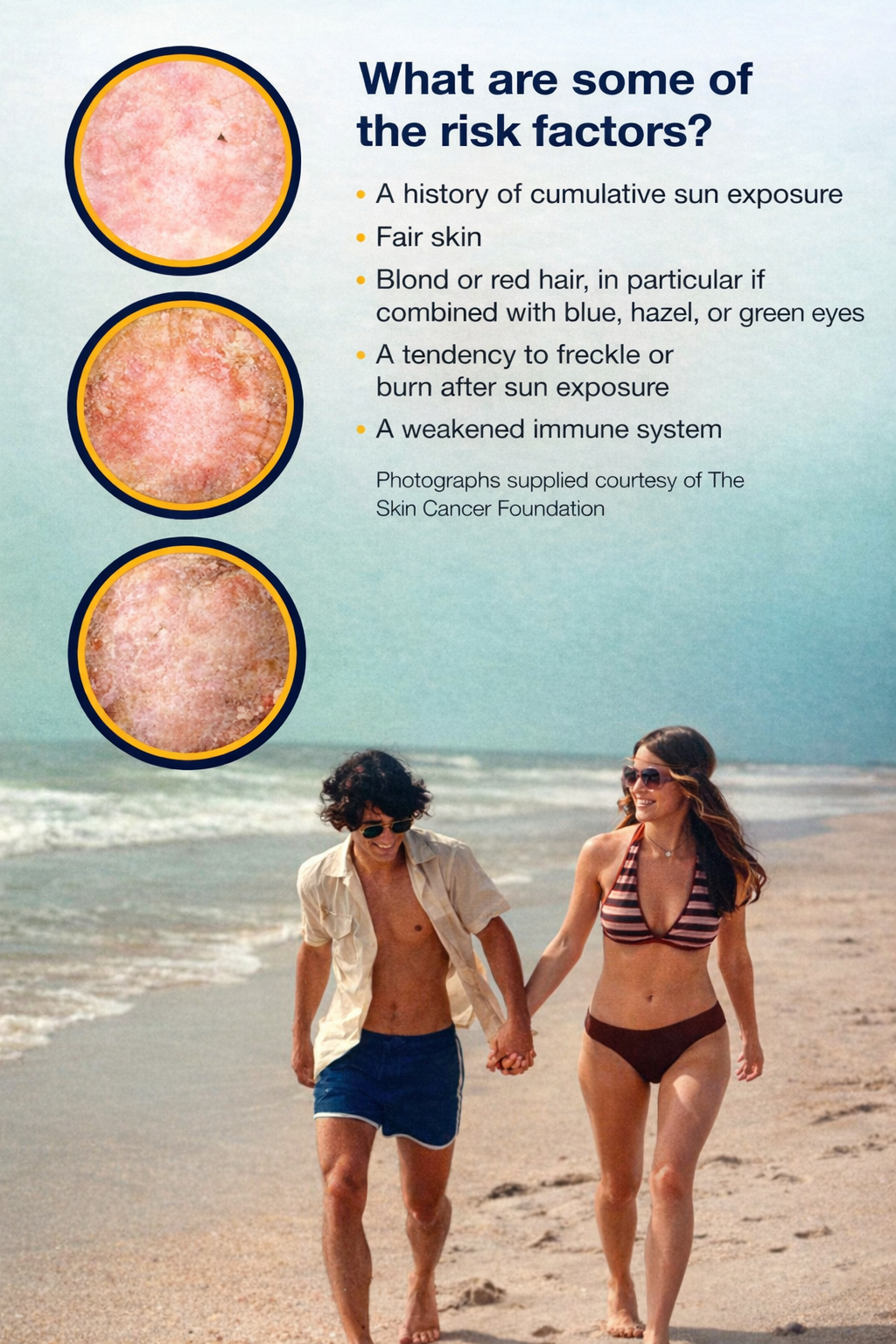
What are Actinic Keratoses?
Actinic keratoses (AKS) are rough-textured, dry, scaly, crusty patches on the skin that are caused by long term exposure to ultraviolet light (UV), such as sunlight. If untreated, AKS may develop into skin cancer.
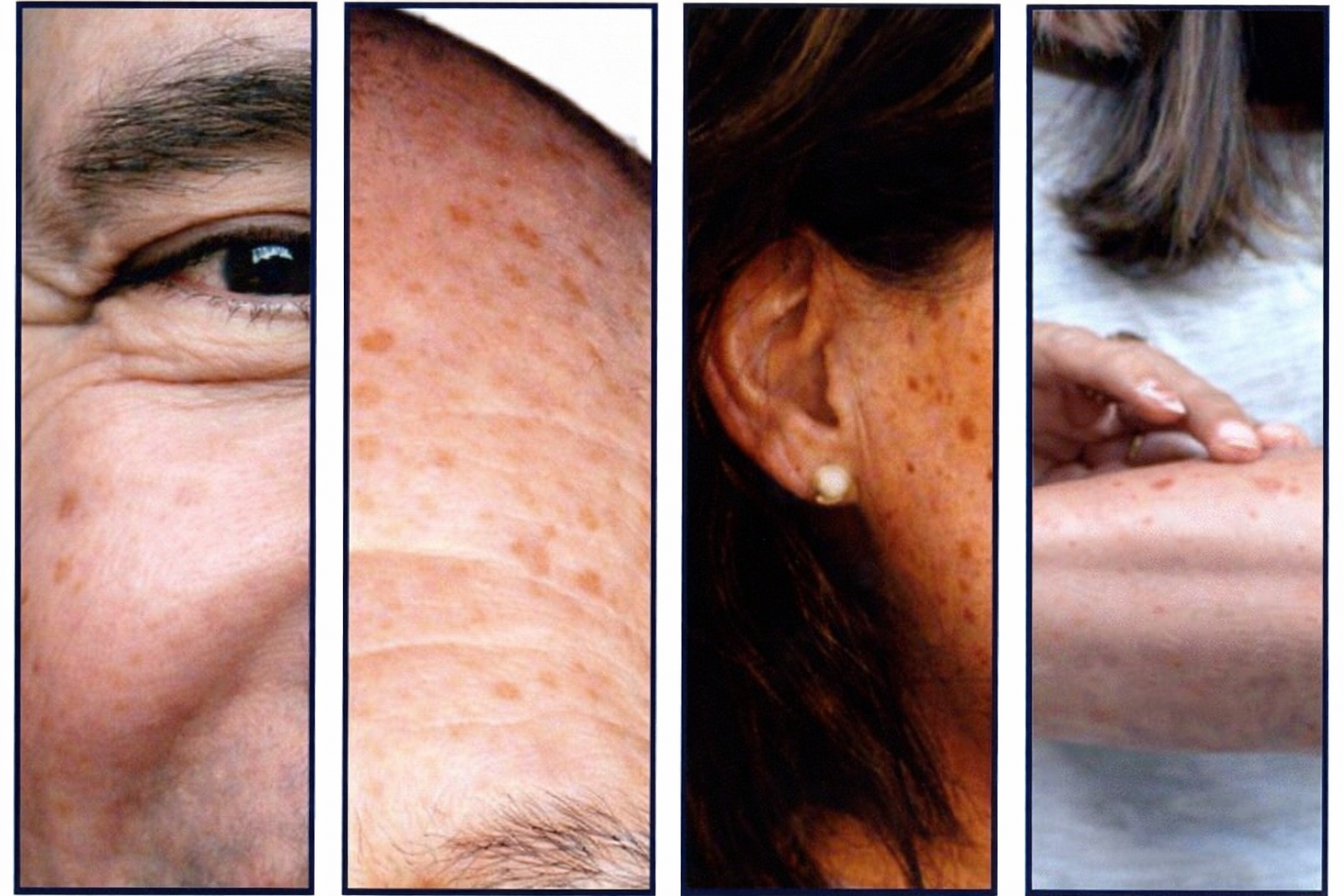
AKS are often called "sun spots"
There may be a single AK or multiple AKS
They can be as small as a pinhead or larger than a quarter
They can range in color from skin toned to reddish brown
Who gets Actinic Keratoses
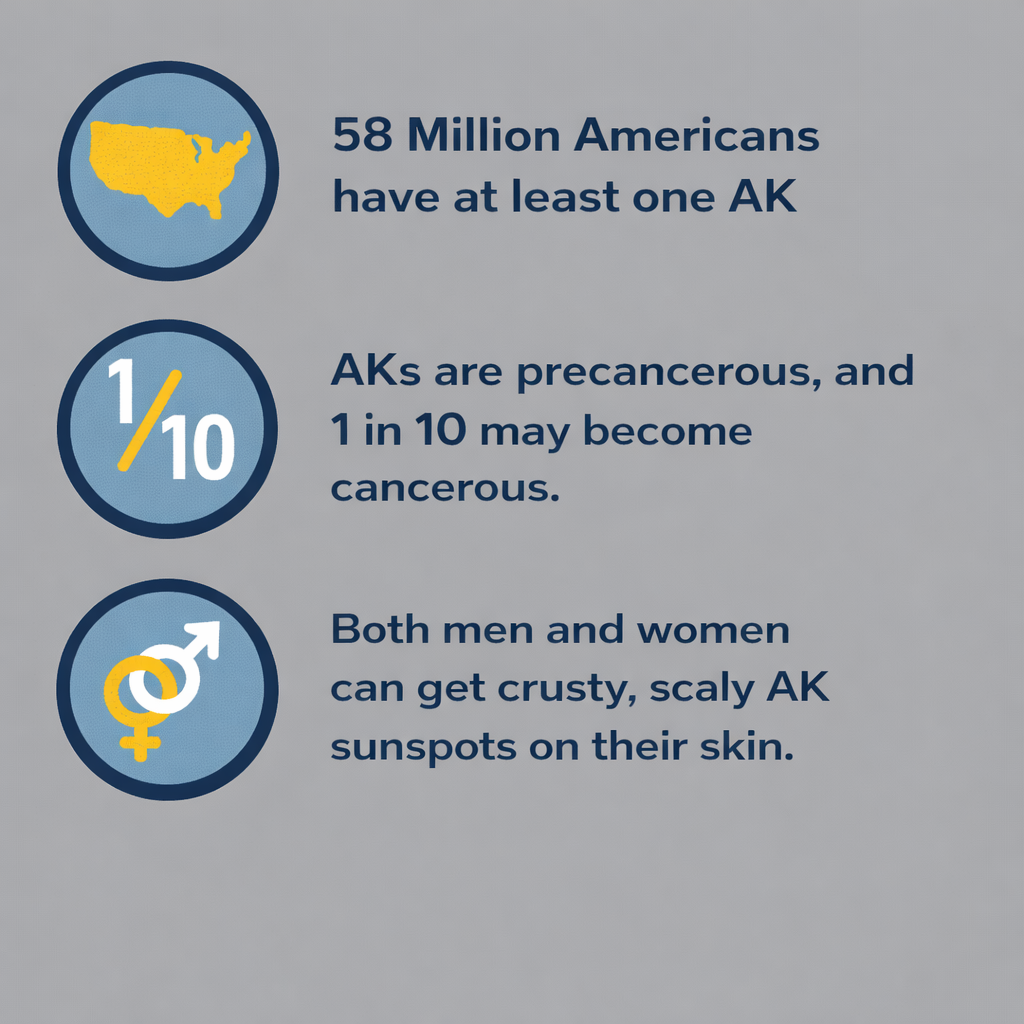
58 million Americans have AK sunspots. Even if you didn't sunbathe much, years of just doing simple tasks outside like playing sports, walking the dog or driving in your vehicle can add up to a significant amount of sun exposure.
Know Before you go...
Before treatment, it's important to let your doctor know if you're taking any oral medications or using any prescription or nonprescription products on your face, head, arms, or hands. There are also a few steps you'll need to take after your treatment:
Avoid exposing treated areas to sun or any bright light for 40 hours.
Wear a hat or long-sleeved shirt to cover treated areas if you go outside.
Expect any redness and swelling to subside after 4 weeks on your face or head or 8 weeks on your arms or hands. The most common side effects after treatment are all temporary. They include stinging, burning, redness, swelling, scaling/ crusting, itching, erosion, oozing, and dryness.
IMPORTANT SAFETY INFORMATION
LEVULAN KERASTICK may cause serious side effects, including temporary memory problems: Temporary memory problems have happened during treatment with LEVULAN KERASTICK in combination with BLU-U Blue Light Photodynamic Therapy Illuminator.
Ask your doctor if LEVULAN KERASTICK 20% solution + BLU-U blue light is right for you.!
A Convenient in-office Treatment Covered by Medicare and Most Commercial Insurance Plans
2-step treatment procedure that can be completed
Within 24 hours
Low downtime
Administered by a qualified healthcare professional
Typically no pharmacy visit is required
No daily medication to apply
Flexible treatment for a range of minimally to moderately
Thick AKs on the face, scalp, or upper arms
IMPORTANT SAFETY INFORMATION
You or your family members or caregiver should call your healthcare provider right away if you develop any problems with memory, confusion, or disorientation during treatment.
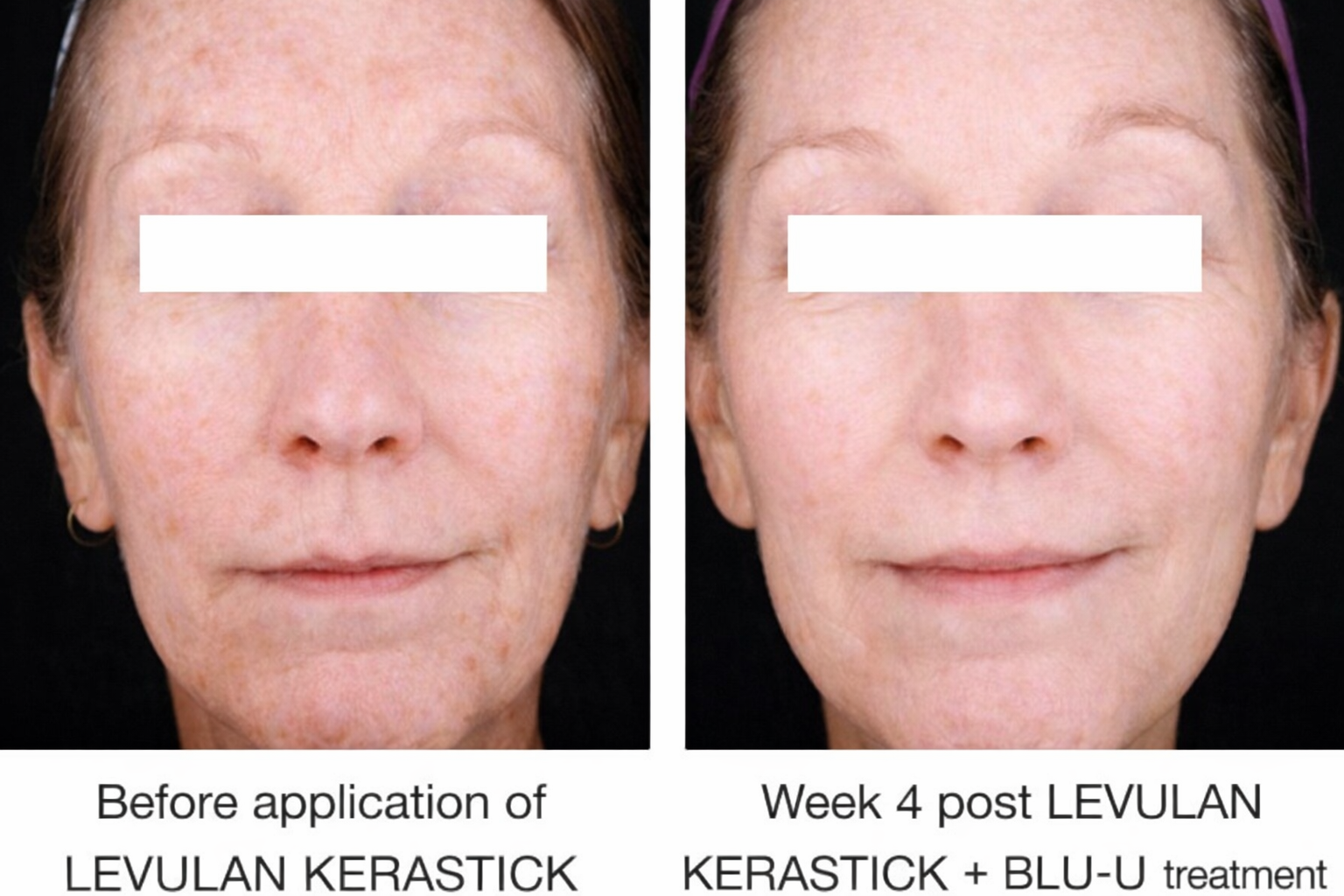
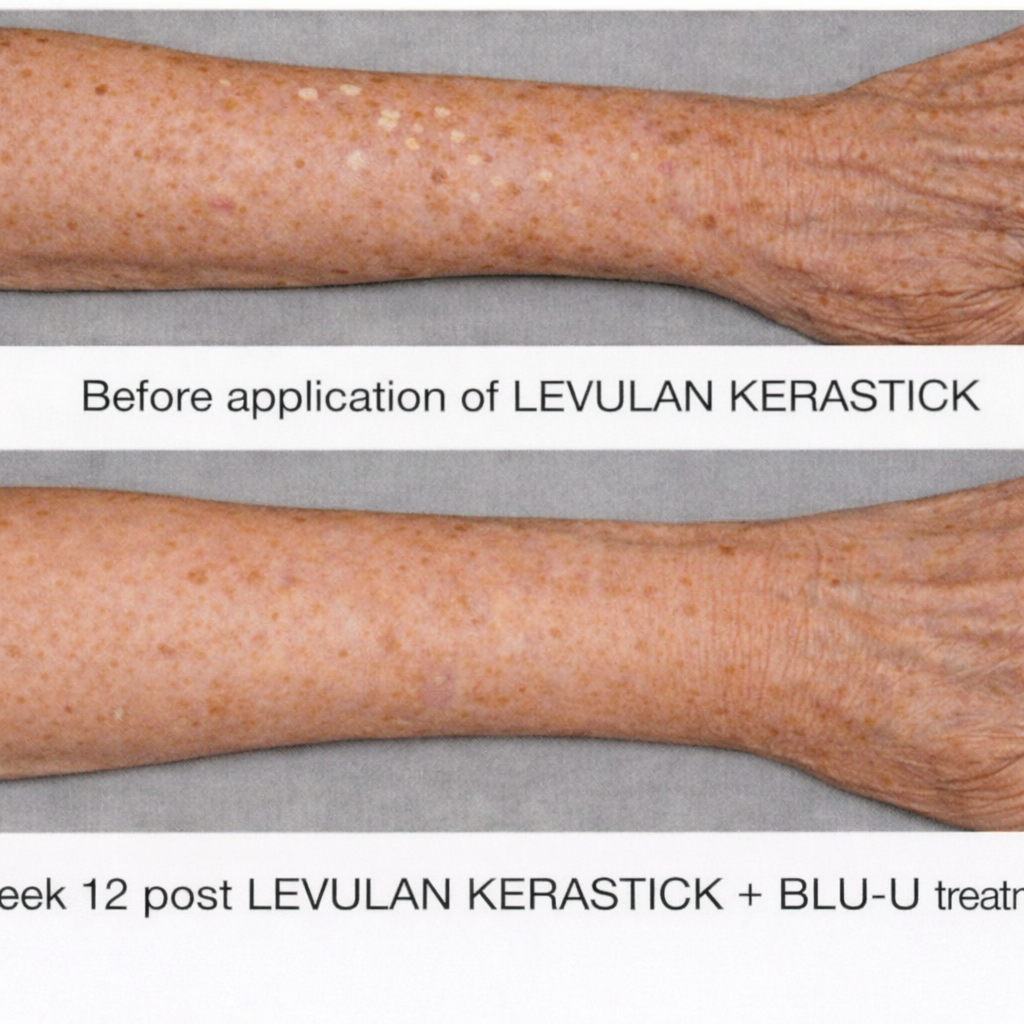
Healing Begins When Treatment Ends..
Patients following 1 treatment with LEVULAN KERASTICK + BLU-U.
IMPORTANT SAFETY INFORMATION
LEVULAN KERASTICK may cause serious side effects, including sensitivity to light and skin irritation. LEVULAN KERASTICK topical solution contains alcohol and may cause skin irritation if covered 9 or bandaged for longer than 3 hours. LEVULAN KERASTICK topical solution contains alcohol and may cause skin irritation if covered 9 or bandaged for longer than 3 hours.
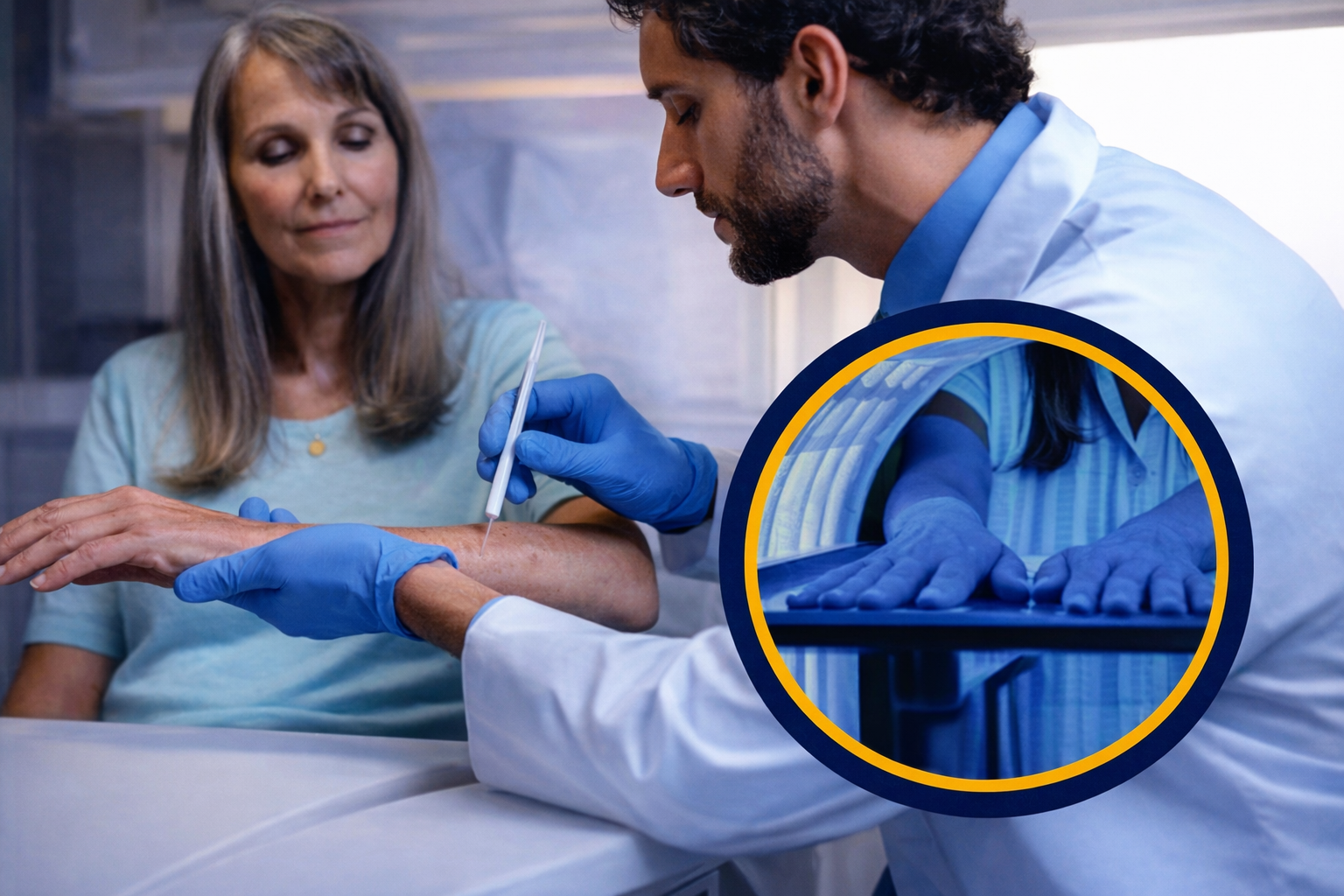
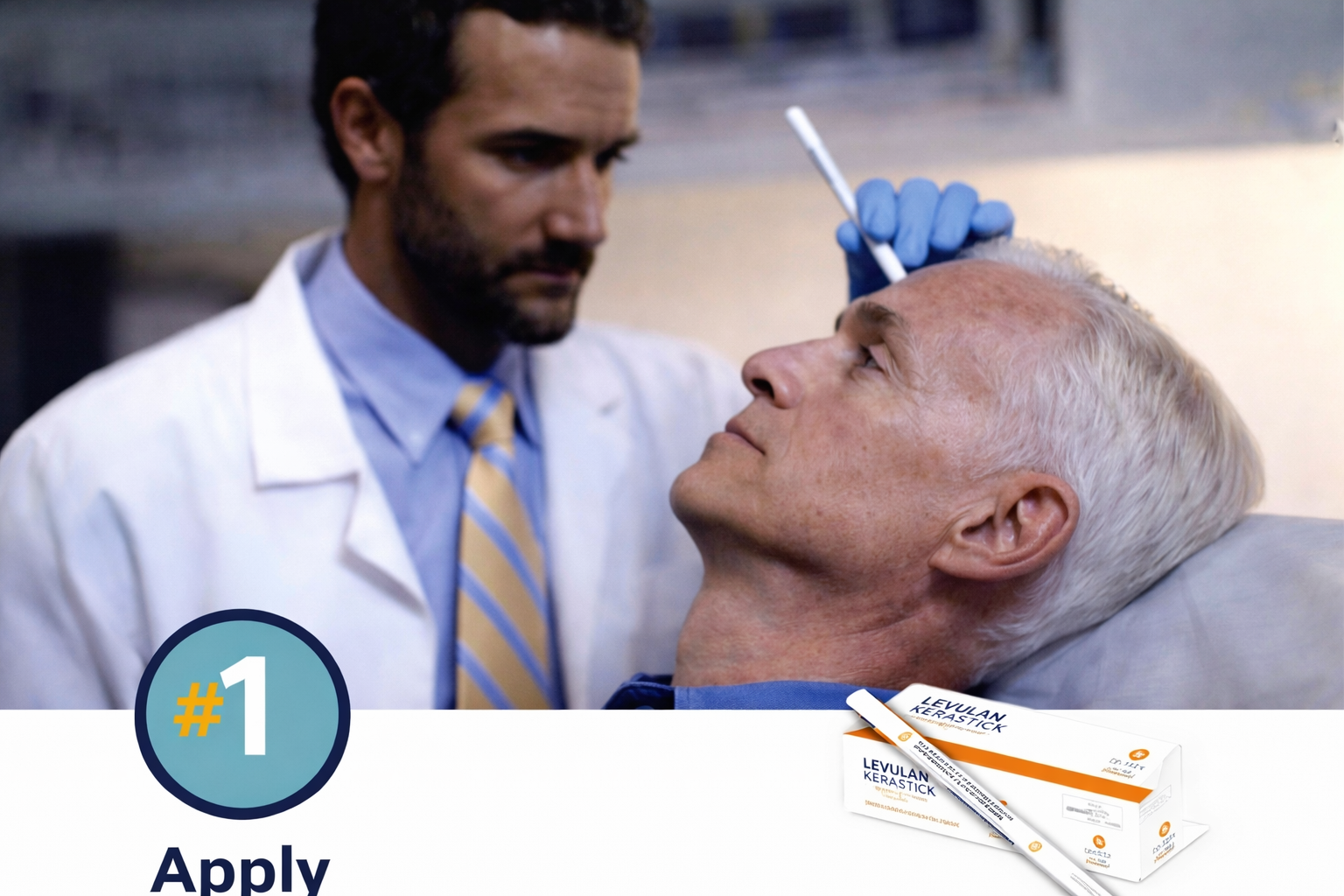
Your dermatologist will apply LEVULAN KERASTICK 20% solution to your AKs.
The solution needs time to properly absorb into your skin.
The waiting period is 3 hours for AKs on upper arms, forearms, and hands and 14–18 hours for AKs on the face or scalp.
IMPORTANT SAFETY INFORMATION
After LEVULAN KERASTICK topical solution is applied to your skin you should avoid sunlight or bright indoor light (such as examination lights, operating room lights, tanning beds, or lights that are close to you) for 40 hours. During this time, the treated areas of your skin will become sensitive to light.
LEVULAN KERASTICK + BLU-U
LEVULAN KERASTICK + BLU-U is a 2-step in-office (medication + blue light) treatment designed to treat minimally to moderately thick actinic keratoses (AK) lesions on the face, scalp, or upper arms.
To activate the LEVULAN KERASTICK 20% solution, you'll sit or stand next to the BLU-U blue light Illuminator for 16 minutes and 40 seconds. Your dermatologist will give you protective eye wear during this step.You can resume your normal activities afterward. Keep treated areas out of the sun and away from bright light for 40 hours.
IMPORTANT SAFETY INFORMATION
Do not receive LEVULAN KERASTICK treatment if you:
Are allergic to aminolevulinic acid HCI or to any of the ingredients in LEVULAN KERASTICK.
Have porphyria or are allergic to porphyrins
Have a skin sensitivity to blue light.
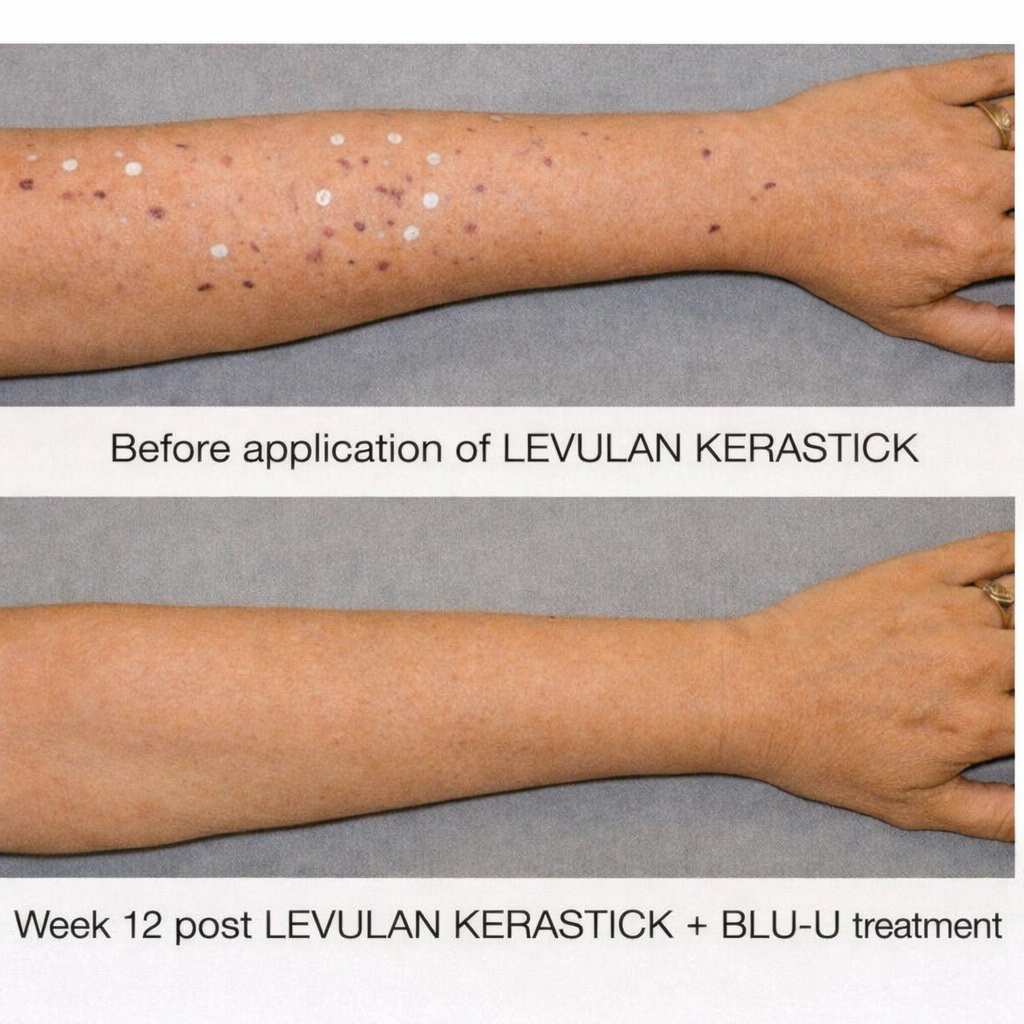
Patients following 2 treatments with
LEVULAN KERASTICK + BLU-U.
Before treatment the perimeter of each AK lesion was marked with 3 dots and then labeled with a numbered label. At follow-up visits, lesions that were cleared were not marked or numbered.
Proven results for millions of patients
As the first and only PDT approved to treat the face and scalp as well as the upper arms, forearms, and hands, LEVULAN KERASTICK 20% solution + BLU-U blue light has been trusted and proven for over 20 years.
✔ 100% clearance: Most patients get 100% clearance of AK sunspots with 1–2 in-office treatments
✔ Long-lasting results: Most face or scalp AKs remain 100% clear 1 year after the first treatment, with no reported scars
✔ Treat even more AKs: This treatment covers larger areas of your body than other PDTs, so you can have multiple AK sunspots treated at once
✔ Focused on safety: The most common side effects are stinging, burning, redness, and itching where LEVULAN KERASTICK was applied
✔ Covered by Medicare and most commercial insurance plans: Confirm coverage details with your provider and insurance company before your treatment
Why treatActinic Keratoses?
Actinic keratoses are the most common precursor to skin cancer
AKs may be found anywhere on the body that has been exposed to UV radiation. They are most commonly found on the face, scalp, ears and arms.
AKs are considered to be the first step in the development of skin cancer. They have the potential to progress to squamous cell carcinoma (SCC). While most AKs remain benign, studies have shown that the majority of SCC arises from AK lesions.¹
Since there is no way to know ahead of time which AKs will develop into SCC, it is very important for individuals with AKs to be under a dermatologist’s care.
Frequent skin examinations may help early detection and prevention.
IMPORTANT SAFETY INFORMATION
LEVULAN KERASTICK (aminolevulinic acid HCl) is for use only as an in-office treatment. LEVULAN KERASTICK treatment is given by a healthcare provider only and is not for use at home.
What is LEVULAN KERASTICK?
LEVULAN KERASTICK is a prescription medicine used on the skin with blue light treatment (BLU-U Blue Light Photodynamic Therapy or PDT) for the treatment of minimally to moderately thick actinic keratoses (AKs) of the face, scalp, or upper arms.
It is not known if LEVULAN KERASTICK is safe and effective in children under 18 years of age.
Who should not receive LEVULAN KERASTICK treatment if you:
• Are allergic to aminolevulinic acid HCl or any of the ingredients in LEVULAN KERASTICK
• Have porphyria or are allergic to porphyrins
• Have a skin sensitivity to blue light
Before receiving LEVULAN KERASTICK treatment, tell your healthcare provider about all of your medical conditions, including if you:
• Have blood clotting problems
• Are pregnant or plan to become pregnant. It is not known if LEVULAN KERASTICK will harm your unborn baby
• Are breastfeeding or plan to breastfeed. It is not known if LEVULAN KERASTICK passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during LEVULAN KERASTICK treatment
• Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LEVULAN KERASTICK and other medicines may affect each other.
IMPORTANT SAFETY INFORMATION CONTINUED…
How will I receive LEVULAN KERASTICK treatment?
LEVULAN KERASTICK treatment is received in two parts:
Your healthcare provider will apply LEVULAN KERASTICK topical solution to your skin lesions. You should not wash the treated areas before you return to your healthcare provider for blue light treatment.
After the prescribed amount of time you will return to your healthcare provider for blue light treatment. If you cannot return for blue light treatment on schedule, avoid sunlight and bright indoor light for at least 40 hours after LEVULAN KERASTICK topical solution has been applied.
During blue light treatment, you will likely feel tingling, stinging, prickling, or burning of the treated areas.
What should I avoid during LEVULAN KERASTICK treatment?
After LEVULAN KERASTICK topical solution is applied to your skin you should avoid sunlight or bright indoor light (such as examination lights, operating room lights, tanning beds, or lights that are close to you) for 40 hours. During this time, the treated areas of your skin will become sensitive to light.
Exposure to light during this time may cause you to feel a burning or stinging sensation and may cause your treated lesions to become red or swollen. You should wear appropriate protective apparel such as a wide-brimmed hat, long sleeve shirt, and gloves to protect your treated skin from sunlight and other bright light.
Sunscreen will not protect the treated areas of your skin against sensitivity to light.
What are the possible side effects of LEVULAN KERASTICK?
LEVULAN KERASTICK may cause serious side effects, including:
Temporary memory problems: Temporary memory problems have happened during treatment with LEVULAN KERASTICK in combination with BLU-U Blue Light Photodynamic Therapy illuminator. You or your family members or caregiver should call your healthcare provider.